Abstract
This guideline covers the wide range of global efforts to counter the COVID-19 pandemic, aiming to outline options for the delivery of care.
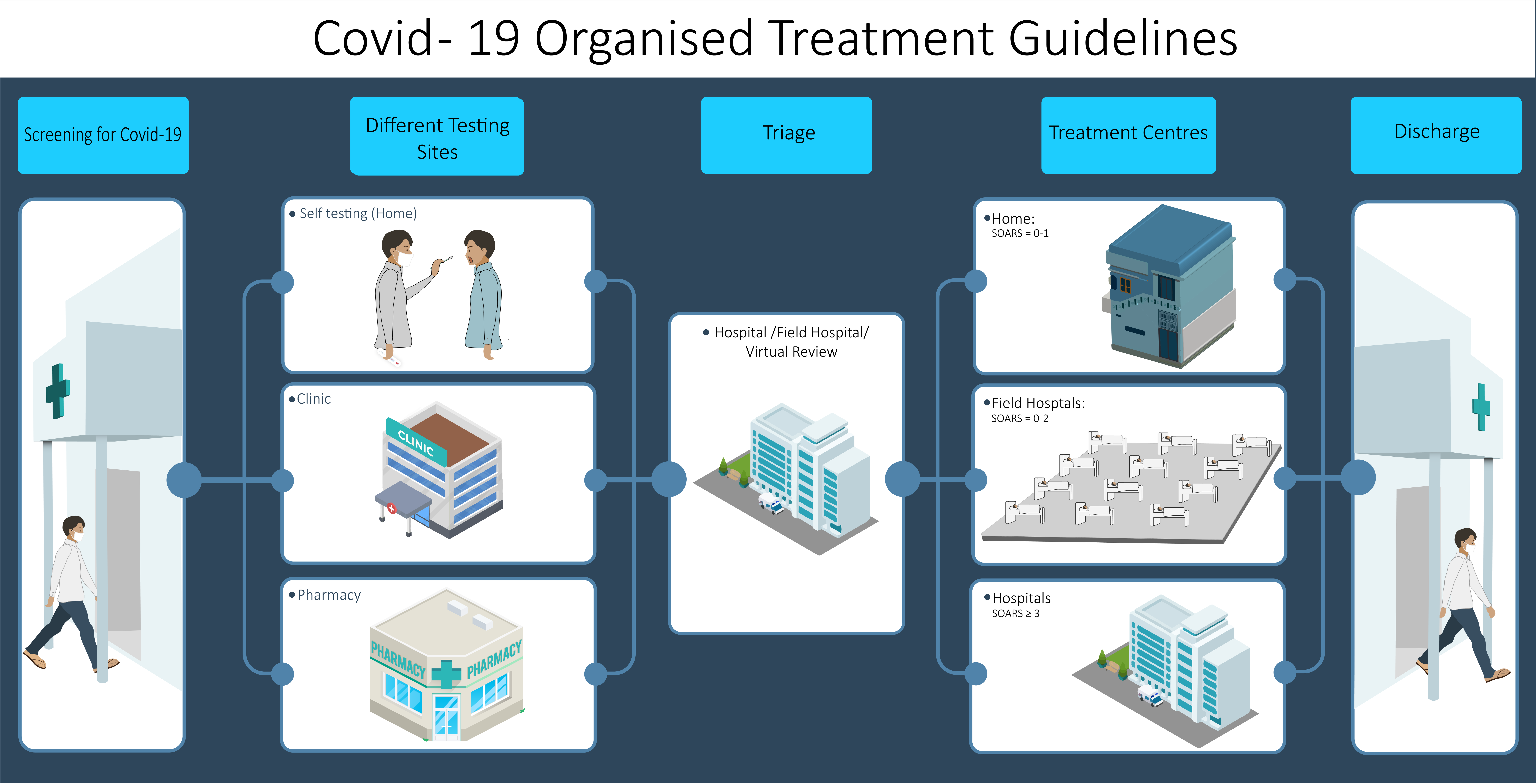
In noting the wide variation in testing, triage, management and vaccination (summarised in Table 1.1–5 ), we seek to identify opportunities for rapid transformational change particularly in countries with stretched resources and less well-developed healthcare systems.
The infographics should enable easy translation, education, and dissemination among both the healthcare population and the public. It has been divided into organisational delivery, treatment, and vaccination.
References
REFERENCES
Technical guidance publications. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications.
Kolb, M., Dinh-Xuan, A. T. & Brochard, L. Guideline-directed management of COVID-19: Do’s and Don’ts. European Respiratory Journal 57, 2100753 (2021).
Welte, T. et al. Current evidence for COVID-19 therapies: A systematic literature review. European Respiratory Review vol. 30 (2021).
Chalmers, J. D. et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. European Respiratory Journal 57, 2100048 (2021).
NHS England and NHS Improvement coronavirus. Coronavirus guidance for clinicians and NHS managers. NHS England Web Site (2020).
Hopkins, J. COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center 1 https://coronavirus.jhu.edu/map.html%0Ahttps://coronavirus.jhu.edu/data/mortality%0Ahttps://coronavirus.jhu.edu/map.html (2020).
COVID Live Update: 160,759,152 Cases and 3,337,893 Deaths from the Coronavirus - Worldometer. https://www.worldometers.info/coronavirus/.
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. The BMJ 369, m1985 (2020).
Grasselli, G. et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - Journal of the American Medical Association 323, 1574–1581 (2020).
Chaudhry, R., Dranitsaris, G., Mubashir, T., Bartoszko, J. & Riazi, S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes-NC-ND license. (http://creativecommons.org/licenses/by-nc-nd/4.0/). (2020) doi:10.1016/j.eclinm.2020.100464.
Koo, J. R. et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. The Lancet Infectious Diseases 20, 678–688 (2020).
New Zealand COVID: 2,645 Cases and 26 Deaths - Worldometer. https://www.worldometers.info/coronavirus/country/new-zealand/.
Robert, A. Lessons from New Zealand’s COVID-19 outbreak response. The Lancet Public Health vol. 5 e569–e570 (2020).
Jefferies, S. et al. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. The Lancet Public Health 5, e612–e623 (2020).
Hsiang, S., Allen, D., Annan-Phan, S. & al., et. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature 584, 262–267 (2020).
Ng, Y., Li, Z., Chua, Y. & al., et. Evaluation of the effectiveness of surveillance and containment measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb Mortal Wkly Rep 69, 307–311 (2020).
Wright, L., Steptoe, A. & Fancourt, D. Trajectories of compliance with COVID-19 related guidelines: longitudinal analyses of 50,000 UK adults. medRxiv 2021.04.13.21255336 (2021) doi:10.1101/2021.04.13.21255336.
Hills, S. & Eraso, Y. Factors associated with non-adherence to social distancing rules during the COVID-19 pandemic: a logistic regression analysis. BMC Public Health 21, 1–25 (2021).
COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/.
Schmidt, M. et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Medicine 47, 60–73 (2021).
Grasselli, G. et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - Journal of the American Medical Association 323, 1574–1581 (2020).
Aziz, S. et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Medicine (2020) doi:10.1007/s00134-020-06092-5.
Leshem, E. & Wilder-Smith, A. COVID-19 vaccine impact in Israel and a way out of the pandemic. Lancet (London, England) 0, (2021).
Haas, E. J. et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet (London, England) 0, (2021).
Kamath, S., Kamath, R. & Salins, P. COVID-19 pandemic in India: Challenges and silver linings. Postgraduate Medical Journal vol. 96 422–423 (2020).
Guglielmi, G. Rapid coronavirus tests: a guide for the perplexed. Nature 590, 202–205 (2021).
Marshall, J. C. et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases vol. 20 e192–e197 (2020).
Chua, F. et al. Early prognostication of COVID-19 to guide hospitalisation versus outpatient monitoring using a point-of-test risk prediction score. Thorax (2021) doi:10.1136/thoraxjnl-2020-216425.
Francis, N. A. et al. Predictors of clinical deterioration in patients with suspected COVID-19 managed in a virtual hospital’ setting: A cohort study. BMJ Open 11, 45356 (2021).
Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. The BMJ 370, 22 (2020).
NHS England » COVID Oximetry @home. https://www.england.nhs.uk/nhs-at-home/covid-oximetry-at-home/.
Ghani, H. et al. Evaluation of the ROX index in SARS-CoV-2 Acute Respiratory failure treated with both High-Flow Nasal Oxygen (HFNO) and Continuous Positive Airway Pressure (CPAP). medRxiv 2021.03.23.21254203 (2021) doi:10.1101/2021.03.23.21254203.
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology vol. 20 363–374 (2020).
Shi, Y. et al. COVID-19 infection: the perspectives on immune responses. Cell Death and Differentiation vol. 27 1451–1454 (2020).
Weinreich, D. M. et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. New England Journal of Medicine 384, 238–251 (2021).
Katz, L. M. (A Little) Clarity on Convalescent Plasma for Covid-19. New England Journal of Medicine 384, 666–668 (2021).
Asthma drug budesonide shortens recovery time in non-hospitalised patients with COVID-19 — PRINCIPLE Trial. https://www.principletrial.org/news/asthma-drug-budesonide-shortens-recovery-time-in-non-hospitalised-patients-with-covid-19.
Ramakrishnan, S. et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. The Lancet Respiratory Medicine 0, (2021).
COVID-19 Studies from the World Health Organization Database - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/who_table.
Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1.
National Institute for Health and Care Excellence. (2021). Therapeutics for COVID-19. [COVID-19 rapid guideline: Managing COVID-19]. https://app.magicapp.org/#/guideline/L4Qb5n/rec/LwomXL (2021).
Therapeutic Management | COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/.
Chalmers, J. D. et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. European Respiratory Journal 57, 2100048 (2021).
CLINICAL GUIDANCE FOR MANAGEMENT OF ADULT COVID-19 PATIENTS | AIIMS Covid Information Portal. https://covid.aiims.edu/clinical-guidance-for-management-of-adult-covid-19-patients/.
Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020).
Abu-Raddad, L. J., Chemaitelly, H., Butt, A. A. & National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. The New England journal of medicine (2021) doi:10.1056/NEJMc2104974.
Skowronski, D. & de Serres, G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine (2021) doi:10.1056/nejmc2036242.
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. www.thelancet.com 397, 2021 (2021).
Emary, K. R. W. et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. www.thelancet.com 397, (2021).
Madhi, S. A. et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine (2021) doi:10.1056/nejmoa2102214.
Use of the AstraZeneca COVID-19 (AZD1222) vaccine: updated JCVI statement, 7 May 2021 - GOV.UK. https://www.gov.uk/government/publications/use-of-the-astrazeneca-covid-19-vaccine-jcvi-statement-7-may-2021/use-of-the-astrazeneca-covid-19-azd1222-vaccine-updated-jcvi-statement-7-may-2021.
Voysey, M. et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. The Lancet 397, 881–891 (2021).
AZD1222 US Phase III primary analysis confirms safety and efficacy. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html.
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 384, 403–416 (2021).
Thompson, M. G. et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. MMWR. Morbidity and Mortality Weekly Report 70, 495–500 (2021).
Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials | Novavax Inc. - IR Site. https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0.
Shinde, V. et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine NEJMoa2103055 (2021) doi:10.1056/NEJMoa2103055.
Administration, D. Janssen COVID-19 Vaccine EUA Letter of Authorization. (2021).
Sadoff, J. et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. The New England journal of medicine (2021) doi:10.1056/NEJMoa2101544.
FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review | FDA. https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough.
Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use | Johnson & Johnson. https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright (c) 2021 Array